
Despite the challenges of 2021, ANZGOG’s clinical research activity has grown. Our members and staff have shown resilience and determination, delivering a busy trial portfolio along with membership growth with a focus on a diverse portfolio of clinical trials in gynaecological cancer.
Endometrial cancer was a priority in 2021. We opened the AtTEnd international study in Australia and implemented ANZGOG’s new endometrial cancer research initiative, EDEN. The catalyst for EDEN was an Endometrial Cancer Research Workshop with researchers attending from across Australia and New Zealand, representing surgical, medical oncology, radiation oncology, quality of life and pre-clinical and translational researchers, as well as cancer consumers. A Steering Committee has now been established and will be leading the development of the next stage of the EDEN initiative.
ANZGOG’s ovarian cancer trials continued to grow with the SOLACE2 and IGNITE studies achieving their 50% recruitment milestones, whilst the exercise intervention study ECHO was awarded $2m by Cancer Council Queensland’s Accelerating Collaborative Research Program.
We are committed to sharing outcomes of our research to help drive improvements in clinical practice. The ANZGOG-led international cervical cancer study, OUTBACK, was selected for a plenary session at the American Society of Clinical Oncology’s (ASCO) 2021 Annual Meeting. The study results were negative but highlighted a need for change in clinical practice in many countries participating in the study and were a focal point for the ASCO communication in 2021.
ANZGOG ‘s translational research initiative TR-ANZGOG was officially launched by Professor Anna DeFazio at ANZGOG’s virtual Annual General Meeting on 22 October 2020.
Our 2021 Virtual Annual Scientific Meeting was our most attended event to date, with a total of 340 registrations. It was a successful, stimulating event made by the enormous efforts and contributions of many.
The volunteers of the Survivors Teaching Students program pivoted to virtual during COVID-19 and continued to be a force to be reckoned with. The 100+ volunteers shared their personal stories on diagnosis and care to health professional students at Universities across Australia.
Public support through donations and partnerships has been well maintained through 2021 with WomenCan and Team Teal engaging the community to increase vital awareness of gynaecological cancer and raise funds to support ANZGOG’s research projects.
I wish to thank all of the members, patients and our staff for another successful year of gynaecological cancer research in Australia and New Zealand, helping ANZGOG to improve life for women with a gynaecological cancer.















AtTEnd, an endometrial cancer trial, opens to recruitment in Australia.

Save the Box September – fundraising and awareness campaign – held throughout Gynaecological Cancer Awareness Month, including contributions and support from Australian artists.

Endometrial cancer workshop held, where 61 attendees discussed issues and shared ideas for how best to improve outcomes.

TR-ANZGOG, ANZGOG’s world-class translational research initiative, officially launches at the ANZGOG 2020 Annual General Meeting, achieving a strategic milestone.

Exercise-intervention trial - ECHO - receives a $2m grant from Cancer Council QLD through Griffith University to expand the study.

ANZGOG hosts Cervical & Vulvar Preceptorships, with 98 fellows, trainees, registrars, and early career clinicians attending virtually.

As part of its member education activities, ANZGOG presented two webinars - Ovarian Cancer Systems of Care. Supported by AstraZeneca, the webinars were attended by 229 health professionals from ANZ, Asia and Europe.

ANZGOG Director Alison Brand appointed Member of the Order of Australia for her significant service to gynaecology, medicine and medical organisations in the Australia Day awards.

ANZGOG hosted its first Virtual Annual Scientific Meeting - delivering an interactive and meaningful program to 354 delegates – researchers, clinicians, nurses, study coordinators and those with an interest in gynaecological cancer research.
WomenCan’s annual Team Teal campaign raises over $371,000 through 429 reinswoman wins, pledges, sponsorships, merchandise sales and community fundraising.

ANZGOG Director Alison Brand AM appointed Chair-Elect to the Gynecological Cancer InterGroup (GCIG), demonstrating ANZGOG’s global presence in the gynaecological oncology space.

*Endometrial cancer study – PHAEDRA – has its results published by the Journal for ImmunoTherapy of Cancer.

A significant international study in cervical cancer treatment, the ANZGOG-led OUTBACK trial was presented at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting Plenary Session to reveal its practice-changing results.

OASIS study, IGNITE, receives support to expand recruitment to the study.


Translational ANZGOG, ‘TR-ANZGOG’, is an exciting ANZGOG research initiative established to achieve a 2018 ANZGOG Strategic Goal: “World-class translational research in gynaecological cancers”.
The TR-ANZGOG research initiative achieved a key milestone this year, launching at the ANZGOG AGM in October 2020 with a presentation by TR-ANZGOG Steering Committee Chair, Professor Anna DeFazio and TR-ANZGOG Project Manager, Claire Davies.
Coinciding with this launch, the TR-ANZGOG Information and Resource Portal, available through ANZGOG’s website, went live for ANZGOG members.
The key elements needed to integrate TR-ANZGOG into future ANZGOG trials have now been established. ITTACc will be the first ANZGOG trial to implement TR-ANZOG with the first TR-ANZGOG participants anticipated early 2022, commencing establishment of an important resource to enable future translational research.
Another significant milestone was the approval of the first TR-ANZGOG Network Laboratories. TR-ANZGOG Network Laboratories will help process, store and distribute samples generously donated by women participating in ANZGOG trials, to maximize both the woman’s participation, and the financial investment made in the trial.

“It was a pleasure to finally launch TR-ANZGOG this year, after extensive consultation and a huge amount of work to ensure that the pieces of the puzzle were ready and aligned with what ANZGOG will need for the next-generation of clinical trials, from a translational research perspective.”
Prof Anna DeFazio, TR-ANZGOG Steering Committee Chair
Building on existing grant success, TR-ANZGOG was awarded philanthropic funding through two Perpetual Foundation endowments. ANZGOG would like to acknowledge the Perpetual Foundation - Kevin Darrell Clarke Endowment and the Perpetual Foundation - The Russell Medical Endowment for their generous support of this important program. Funds will be utilised for: procurement and development of information technology solutions for efficient and comprehensive biospecimen tracking and management.
“The TR-ANZGOG initiative has not come a moment too soon, with a raft of new ANZGOG trials due to open, and with the anticipated increased reliance on biospecimens and molecular pathology. TR-ANZGOG will assist investigators with biospecimen aspects of trial protocols, ensuring adequate skills and resources are available to fully leverage the value of biospecimens for translational research.”
Prof Anna DeFazio
The ANZGOG Endometrial Cancer (EDEN) Research Initiative Steering Committee was established in 2020-2021 to consider the best approach to achieve the strategic direction for ANZGOG’s commitment in further developing its endometrial cancer clinical trials portfolio. The EDEN Steering Committee, Chaired by Prof Linda Mileshkin with Deputy Chair Assoc Prof Alison Brand AM, identified 5 key areas of focus for the initiative as a whole:
Each of these areas of focus are being developed through collaborative consultation with a group of 60+ volunteer ANZGOG members.
The Steering Committee will drive the strategy for the research and work closely with the Uterine Tumour Working Group who will assist investigators to develop their studies and will engage ANZGOG members, public, philanthropic and pharma funders, as well as local and global collaborators of ANZGOG to achieve the Initiative’s goals.

Due to positive signalling from an interim analysis, ANZGOG received approval to expand one of IGNITE’s cohorts – the Cyclin E1 over-expressed without gene amplification cohort - doubling the total number of planned patients in that cohort (now 64 patients).
A study in ANZGOG’s OASIS Initiative, IGNITE is a phase II trial testing whether the use of adavosertib will provide clinical benefit to women with recurrent high-grade serous ovarian cancer. The study is open to recruitment at 10 sites across Australia.
ANZGOG drives a series of world-class research programs including OASIS, matching molecular subtypes of ovarian cancer with targeted new therapies designed to improve women’s lives.
The OASIS research model aims to significantly shorten the cycle of clinical testing by:

“ANZGOG’s OASIS provides an essential platform for our researchers and women, who together are focused on improving ovarian cancer outcomes. By speeding translation, and funding the most cost-efficient trials, we ensure that the most exciting drugs will be available for Australian women who so desperately need access to new treatments. ANZGOG’s OASIS provides urgently needed breakthrough data about the next wave of drugs important for targeting ovarian cancer.”
Prof Clare Scott
Chair - OASIS Steering Committee
Patient Recruitment: 367/500
Sites Open: 9/11
Patient Recruitment: 79/110
Sites Open: 18/19
Patient Recruitment: 78/114
Sites Open: 15/15
Patient Recruitment: 16/60
Sites Open: 11/12
Patient Recruitment: 73/75
Sites Open: 10/10
Patient Recruitment: 1/15
Sites Open: 4/6
Patient Recruitment: 23/70
Sites Open: 6/6
Patient Recruitment: 32/60
Sites Open: 4/6
Patient Recruitment: 0/40
Sites Open: 6/7
Patient Recruitment: 55/96
Sites Open: 9/11
Patient Recruitment: 26/40
Sites Open: 15/15
Planned Sample Size: 200-500
Accrual: 74

OUTBACK TRIAL PRESENTED AT GLOBAL STAGE
The ANZGOG-led international study has been regarded as one of the most important cervical cancer research advances in the past year. The trial was presented at the 2021 ASCO Annual Meeting Plenary Session to reveal its highly anticipated results.
OUTBACK is a phase 3, randomised trial of adjuvant chemotherapy after chemoradiation as primary treatment for locally advanced cervical cancer compared to chemoradiation alone. The trial results were described as “immediately practice-changing” by ASCO President Lori J. Pierce, MD, FASTRO, FASCO.
OUTBACK is an academic collaboration of ANZGOG, NHMRC Clinical Trials Centre at the University of Sydney, and NRG Oncology under the auspices of the international Gynecologic Cancer Intergroup (GCIG). The study began in 2011 and recruited 926 women across seven countries.

ANZGOG’s PHAEDRA study examined the activity of the immunotherapeutic agent durvalumab in women with advanced endometrial cancer that were either dMMR or pMMR. Led by Principal Investigator Assoc Prof Yoland Antill, the study was a Phase II clinical trial conducted in Australia and included women with both types of advanced endometrial cancer. Women received an immune therapy known as durvalumab. PHAEDRA recruited 71 patients in Australia.
PHAEDRA’s results were published by the Journal for ImmunoTherapy of Cancer in June 2021.
An abstract about the PHAEDRA study was accepted to be a part of the ASCO 2021 Poster Session – Gynecologic Cancer. The abstract lead author is ANZGOG Member Dr Deborah Smith, Consultant Anatomical Pathologist at Mater Pathology, QLD.
We thank all contributing groups involved in these achievements.

“The encouraging outcomes are consistent with results coming out from other trials that have tested different immunotherapies in endometrial cancer, and certainly implies a change to the way we should consider treating dMMR endometrial cancers, but also points to the need for further research to understand how to improve on the response to immune therapy, particularly in the pMMR cancers.”
Assoc Prof Yoland Antill, Principal Investigator - PHAEDRA

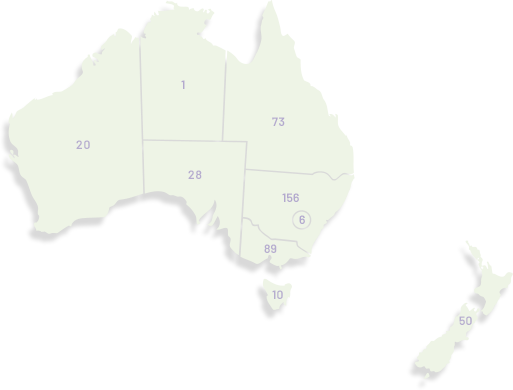
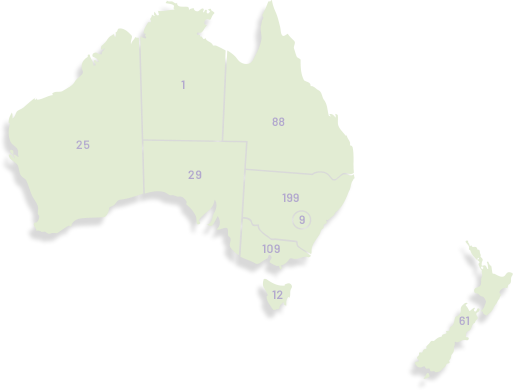
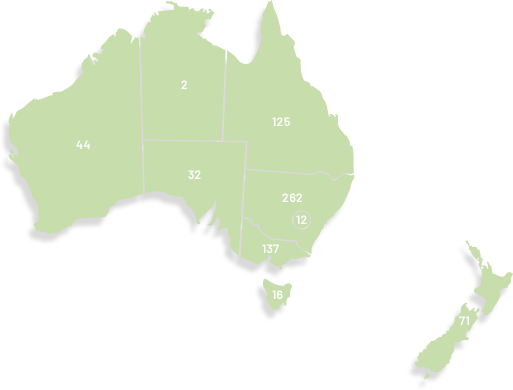

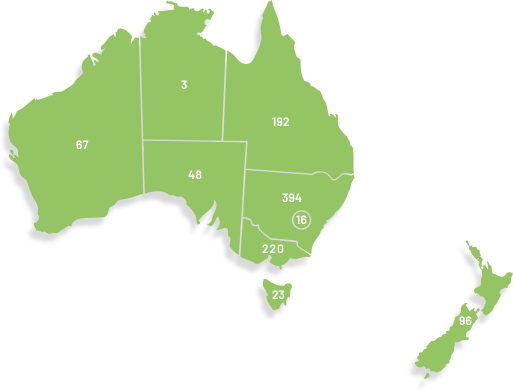
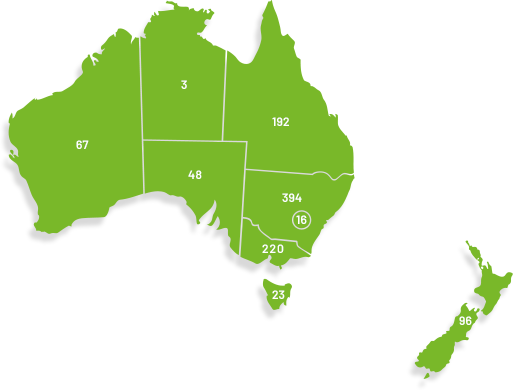
Our membership has grown significantly since our organisation was founded in 2000, doubling in the last seven years to over 1,100. ANZGOG's members in every Australian state and New Zealand are dedicated to growing the research portfolio in both treatment, surgery, radiation oncology, quality of life and survivorship.
Together with our staff, donors and partners, our members work to improve life for women through cancer research.
With conferences being virtual, CRP members attended ANZGOG’s ASM, including a workshop on Quality of Life (QoL) & Economic Evaluation in Research. Members also attended the COSA Survivorship Conference, “Life after Cancer, Reimagined, Redefined and Rebuilt” with its key topic of “Achieving Equity for all Survivors”. Equity in clinical trials is a core CRP focus.
CRP members took their turn too at presenting with sessions at the ANZGOG Ovarian Cancer Webinar. As subject matter experts we contributed to the development of an online education resource for consumers, supplying video content on the role of the CRP and specialist topics from a consumer perspective.
The EDEN Initiative has been a big focus this year. Our CRP Chair Wanda Lawson is a member of the steering committee and co-chairs the “Funding and Patient Advocacy work group”. Within EDEN, CRP members are also participants in the Survivorship work group.
In addition to our normal reviews of RAC submitted concepts, we have contributed to the Taper Endometrial Cancer Grant application and commented along with a consumer team from Germany on the GCIG developed Survivorship Care Plan for Gynaecological patients.


ANZGOG continued its commitment to developing the next generation of leaders by providing an outstanding education experience in the shape of a cervical and vulvar cancer preceptorship and a two-part ovarian cancer webinar series. The attendees had a unique opportunity to gain a greater understanding of best current clinical practice, hearing from many world leaders in the gynaecological cancer space.
This engagement of younger members ensures that ANZGOG can continue on after the current leaders have left.


The Cervical and Vulval Cancer Preceptorship 2020 was held 27 & 28 November 2020 and Chaired by Dr Michelle Harrison and Dr Peey-Sei Kok.
This was a great opportunity for attendees to understand the landmark studies and discuss clinical practice in these cancers, with experts in Australia and New Zealand, and also an excellent learning opportunity for the Fellows and Trainees who presented, including valuable discussion by the mentors and guest panelists.
A total of 98 fellows, trainees, registrars, and early career clinicians attended the sessions virtually.

“The ANZGOG preceptorships are about more than just learning up-to-date, best practice approaches to the management of gynaecological cancers. They are an opportunity to collaborate, to learn from one another, to advocate for women with gynaecological malignancies, and to become excited about the future of our professions.”
Dr Michael Krasovitsky, Medical Oncologist
ANZGOG, as part of its member education activities, presented a two-part Education Webinar Series for ANZGOG members and others across the health sector.
Chaired by Professor Andreas Obermair and Associate Professor Tarek Meniawy, the webinars featured presentations by leading ANZGOG members - Prof Michael Friedlander AM, Prof Clare Scott, Assoc Prof Phlip Beale, and Dr Paul Cohen, providing updates on the latest developments in diagnosis and genetic testing, medical and surgical interventions, as well as follow-up.
The webinars were attended by 228 members and provided them with a unique opportunity to hear from these experts in the field including a panel discussion after each webinar. This Education Webinar Series was supported by AstraZeneca.

Survivors Teaching Students (STS) is a ground-breaking education program that brings the faces and voices of ovarian and other gynaecological cancer survivors and caregivers into the classrooms of health professional students to teach them about women’s experiences with the diseases.
As STS completes its third year in Australia, the program is now working across five states, NSW, VIC, QLD, WA and SA. Managing COVID-19 lockdowns, our 10 STS volunteer teams across Australia continued sharing their stories via a hybrid model, online (via Zoom) and in-person at some Schools in QLD and WA. Though the focus of STS is ovarian cancer, 2020 saw us expand to include all gynaecological cancers.
“I am beyond proud of how successful the Survivors Teaching Students program has become in such a short time and just how many university students we have reached across Australia. The program has created an incredible community of wonderful caring women and men, passionately raising awareness of ovarian cancer amongst the medical professionals of the future.”

ANZGOG’s Virtual Annual Scientific Meeting (ASM) 2021 was held over two days: Friday 5 February for the Pure Science Symposium, and Friday 12 February for the main conference program. The theme of this year’s ASM was 'From Research to Clinical Practice – Patient-Reported Outcomes in Gynaecological Cancers'.
ANZGOG’s first virtual ASM delivered an interactive and meaningful program to over 350 delegates – researchers, clinicians, nurses, study coordinators and those with an interest in gynaecological cancer research.
The Pure Science Symposium was an excellent opportunity to highlight the variety and significance of work performed by our pre-clinical researchers to better understand gynaecological cancers. It was also an invaluable opportunity for our pre-clinical and clinical researchers to engage and collaborate.
After the postponement of last year’s ASM, we thank our three keynote international speakers who joined us virtually to each deliver outstanding and insightful presentations:

Prof Nicoletta Colombo
(Gynaecological Oncologist, University of Milan, Italy)
Secondary debulking surgery for recurrent ovarian cancer – What I do and why.

Prof Amit Oza
(Medical Oncologist, Princess Margaret Cancer Centre, Toronto, Canada)
Ovarian Cancer Building on BRCA: Where Next?

Prof Wui-Jin Koh
(Radiation Oncologist, National Comprehensive Cancer Network, Pennsylvania, USA)
Defining optimal care for gynaecologic cancers – how do we incorporate patient-reported outcomes into decision making?
All the presentations across the two days of the ASM were exceptional, and in particular ANZGOG congratulates the following award recipients:
Other presentations included an update from Wanda Lawson on behalf of the Consumer Research Panel (CRP), updates from the Principal Investigators of ANZGOG trials currently open to recruitment, and an exciting new research concept put forth by a team of nurses, which we hope to hear more about in the future.
The ASM closed with presentations from Prof Michael Friedlander AM, Prof Madeleine King, and Prof Phyllis Butow, highlighting the progress, challenges, and strategies in integrating Patient-Reported Outcomes (PROs) in gynaecological cancer trials and practice. The discussions during this session highlighted the importance and relevance of the theme of this year’s ASM.
"Absolutely fabulous talk yet again from one of the greats. Always very pragmatic and clinically applicable. The message is follow a decision model when discussing secondary cytoreduction with your patient." 💬 Assoc Prof Orla McNally, Gynaecological Oncologist. #ANZGOG2021ASM
— ANZGOG (@anzgog) February 11, 2021



Gynaecological cancer is a global issue. Connecting with other world leaders in research is critical to improving treatments and outcomes for women with gynaecological cancer across the world.
ANZGOG’s strong international collaborations are fostered through its individual members and organisational membership of the Gynecological Cancer InterGroup (GCIG), which is comprised of 32 member countries. ANZGOG is a significant collaborator internationally in gynaecological cancer clinical trials development and regularly has a large number of members participating at the GCIG meetings and on its various committees.

In the GCIG Spring 2021 meeting, ANZGOG Director and past Chair Assoc Prof Alison Brand AM was appointed Chair-Elect to the GCIG, following in the footsteps of another former ANZGOG Chair – Prof Michael Quinn AM – who also served as GCIG Chair. GCIG Chair appointments are for two years. Alison Brand AM has served on the GCIG Executive for several years and was nominated by ANZGOG as their organisation representative.
In addition, Prof Linda Mileshkin was appointed Co-Chair/Chair-elect of the Endometrial Committee of GCIG. Prof Mileshkin is also an ANZGOG Director and Chair of the newly formed ANZGOG Endometrial Cancer Research Initiative (EDEN Initiative). ANZGOG will now have 5 members in key leadership roles within GCIG in the coming year which is a true testament to ANZGOG's reputation as a global leader in gynaecological cancer research:
These GCIG appointments, in addition to several ANZGOG members who are currently committee and council members of International Gynaecological Cancer Society (IGCS), demonstrate ANZGOG’s growing reputation and leadership in the global gynaecological cancer research space.
One of the inaugural members, ANZGOG participated in the formation of the Asia Pacific Gynecological Oncology Trials group (APGOT) in 2019. The group was formed to focus on collaborative studies in gynaecological cancer within the Asia Pacific region, strengthening capabilities and ensuring a greater range of trials available for women.
ANZGOG has already conducted one study (MOCCA) and is currently operating another (VIP) in cooperation with Singapore as part of the OASIS Research Initiative.

ANZGOG continues to collaborate both nationally and globally with other research organisations as demonstrated in the table below:
The AtTEnd clinical trial is for women with advanced stage or recurrent endometrial cancer and will assess whether the use of the immune therapy atezolizumab is of additional benefit to current, first line chemotherapy combination.
AtTEnd is in collaboration with Mario Negri Gynecology Oncology Group (MaNGO) and the NHMRC CTC and is currently recruiting at various clinical sites around Australia and New Zealand.
ANZGOG has a long-term relationship with the NHMRC Clinical Trials Centre (CTC), at the University of Sydney, who have acted as a sponsor of our larger trials. Currently, ANZGOG has eight open trials in collaboration with the CTC, and a further three in development.
Strong local collaborations are fostered with institutions such as Sydney and Melbourne Universities, Walter & Eliza Hall, QIMR Berghofer Medical Research Institute, Queensland University of Technology and University of Western Australia are ensuring a diverse approach to research ideas and clinical trial development.
ANZGOG also works closely with other cooperative cancer clinical trials groups, the Quality of Life Office, CREST Health Economics to foster new trials and contribute to their development.
WomenCan is the fundraising arm of ANZGOG, funding research and education programs conducted by ANZGOG’s membership. WomenCan’s mission is to engage the community to fund pioneering discoveries that will enable women with a gynaecological cancer to live better and live longer. In a challenging year, we pivoted to focus on core areas of fundraising, that with limited resources could bring the greatest return. Team Teal with the Harness Racing industry continues to gain strength in fundraising and commitment. This year was the first year that WomenCan engaged with the art community as a collective in the bespoke fundraiser Art on a Box, to much interest.




A Gift in Will to ANZGOG can benefit women now and long into the future.
“This year we recognise a considerable bequest donor, who wished to remain anonymous, and to who we offer our deep gratitude. We pay our respect to all the women, who were recognised through a WomenCan memorial this year.”
Karen Livingstone AM, Head of Fundraising and Development | WomenCan
For more information about leaving a legacy gift to ANZGOG’s research, please visit here.


On 1 February, every year in collaboration with the Harness Racing industry in Australia and New Zealand, WomenCan launches the Team Teal campaign.
During this campaign 245 reinswomen wore teal pants, raising funds and promoting support for women with ovarian and other gynaecological cancers. In a combined effort the reinswomen crossed the winning post a tremendous 429 times, triggering donations, pledges, and sponsorships towards the Survivors Teaching Students program and to support patient referral pathways. We also acknowledge the Team Teal community fundraisers who held local events including themed race days, cupcake sales, ladies’ nights out, right down to gold coin collections.
The passion that the Harness Racing community continues to demonstrate in supporting women with ovarian cancer is inspiring and proves that Team Teal is a powerful collaboration between the harness racing community and WomenCan.


September is Gynaecological Cancer Awareness Month, which represented an opportunity to call out the need for greater investment into gynaecological cancer research and to fund ANZGOG’s research, whilst engaging the public and introducing more supporters to WomenCan and ANZGOG.
The Save the Box campaign ran from 1st September – 28th October 2020, where supporters were asked to fundraise in different ways. Art on the Box was the most successful feature of the campaign.
Artists were encouraged to paint on a deconstructed box, which was then donated to WomenCan and sold through a Save the Box Virtual Art Gallery.
A total of 32 artists responded and donated 62 artworks, demonstrating the support from the artist community and creating impactful relationships for future public-facing programs.



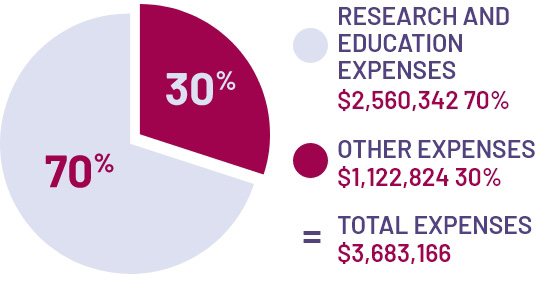

ANZGOG has a collaborative agreement with the University of Sydney which supports the operation of a number of ANZGOG trials by the NHMRC Clinical Trials Centre (CTC), a unit of The University of Sydney which is sponsor of these trials.
Government grant funding for the benefit of ANZGOG clinical trials projects is administered by the University of Sydney and is received annually to support these research activities. Funding received by the University of Sydney for ANZGOG’s research projects, since 2002 totals $21,221,189. These funds are not reported in the ANZGOG Annual Financial Statements but are received for ANZGOG projects by the University of Sydney as a result of our collaboration.
The Cancer Australia Support for Cancer Clinical Trials Program, which is shared 50:50 with the University of Sydney, NHMRC Clinical Trials Centre, provides $500,000 annually and 50% this figure is included in income statements.