RAMP 301 (VS-6766-301/GOG-3097/ENGOT-ov81) is a Phase 3, Randomized, Open-Label Study of Combination Therapy with Avutometinib plus Defactinib Versus Investigator’s Choice of Treatment in Patients with Recurrent Low-Grade Serous Ovarian Cancer (LGSOC).
We’re excited to announce that this trial is now open to recruitment across Australia at the following sites:
- ICON Cancer Centre, QLD. Lead Oncologist: Jeffrey Goh
- Cancer Research, SA. Lead Oncologist: Meena Okera
- Princes of Wales Hospital, NSW. Lead Oncologist: Yeh Chen Lee (Australian Study Chair)
- Sir Charles Gairdner Hospital, WA. Lead Oncologist: Tarek Meniawy
- Peter MacCallum Cancer Center, VIC. Lead Oncologist: Sumitra Ananda
The trial is led by global sponsor Verastem Oncology in partnership with GOG Foundation and European Network of Gynaecological Oncological Trial Groups (ENGOT) and supported in Australia by ANZGOG and local CRO, Catalyst Clinical Research.
Avutometinib is a first-in-class oral RAF/MEK clamp designed to inhibit MEK and block RAF-mediated phosphorylation of MEK2. Avutometinib is being combined with the selective FAK inhibitor defactinib in the RAMP 301 study.
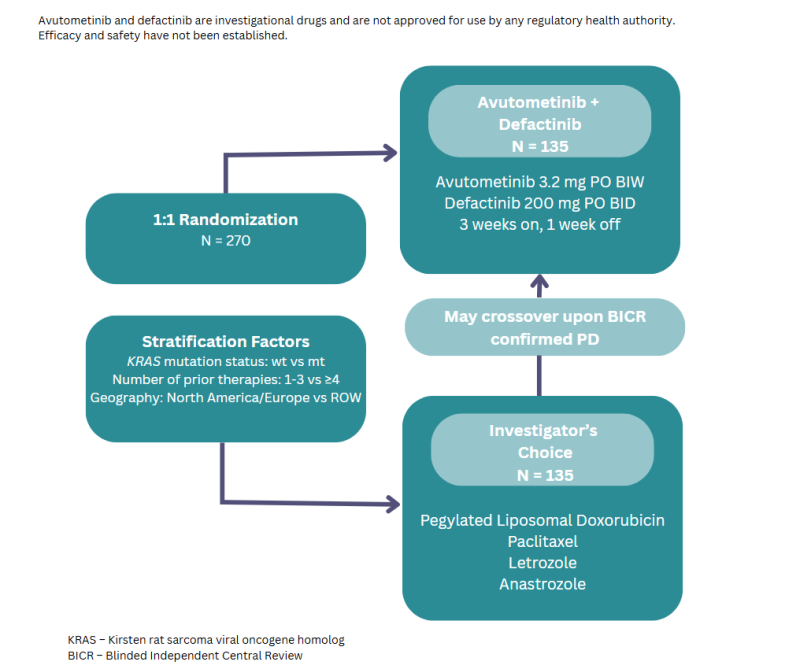
In the study, investigators will evaluate the efficacy and safety of avutometinib and defactinib vs standard of care (SOC) chemotherapy or endocrine therapy in patients with recurrent LGSOC.
RAMP-301 is expected to enrol 270 patients globally in Australia, the USA, Canada, the United Kingdom, Europe, and Korea. The primary endpoint of the study is progression free survival (PFS) by Blinded Independent Central Review. Secondary endpoints include overall response rate, duration of response, disease control rate, safety and tolerability, patient reported outcomes, and overall survival.
RAMP-301 Australian Study Chair

“The RAMP301 is a long awaited trial for our Australian patients with low grade serous ovarian cancer, offering access to avutometinib and defactinib. This international trial is a beacon of hope for patients and investigators, making significant strides forward in delivering treatments that could truly impact the lives of those affected by rare cancers.”
Dr Yeh Chen Lee
Medical Oncologist, NSW
Why is RAMP-301 needed?

This year an estimated 1,786 Australian women will be diagnosed with ovarian cancer.
Low-grade serous ovarian cancer (LGSOC) accounts for approximately 5% of ovarian cancers and is a highly recurrent, chemotherapy-resistant cancer, associated with slow tumour growth and high mortality rate.
LGOSC responds poorly to chemotherapy and mostly affects younger women. They are often treated with ineffective chemotherapy which reduces their quality of life. Recent advances in molecular characterisation of the disease have identified potential targeted therapies that could improve outcomes in these patients.
Eligibility Criteria
The RAMP-301 trial may be suitable for women with recurrent, platinum-resistant, low-grade serous ovarian cancer (ovarian, fallopian, peritoneal).
It is advisable that patients discuss their concerns and the best course of action regarding their participation in clinical trials with their oncologist.
For more information on the RAMP 301 trial and participation criteria please visit the clinical trials page:
Patient referrals to RAMP-301
If you are a physician interested in referring a patient to this study, please contact our ANZGOG Clinical Trial Project Manager at anzgog_ramp301@anzgog.org.au for more information.











